Posted by Colin Hebert, VP Biotechnology & Keya Rodrigues, Quality Assurance Bioengineer
This year’s particularly bad flu season has scientists heavily focused on identifying better ways to produce the influenza vaccine. According to CDC data as of February 17,2018, there was widespread influenza activity across most of North America (1), and one of the contributing factors for the high number of cases might be the reduced effectiveness of H3N2 vaccines produced in eggs (2) (3). “Our experiments suggest that influenza virus antigens grown in systems other than eggs are more likely to elicit protective antibody responses against H3N2 viruses that are currently circulating,” according to Dr. Scott Hensley, PhD, an associate professor of Microbiology, in the Perelman School of Medicine at the University of Pennsylvania (4). This illustrates the importance of cell-based flu vaccine manufacturing processes and the increased flexibility they provide. Quantification of viral infectivity plays a key role in vaccine development, from early stage R&D, to process optimization and scale-up, production monitoring, release assays, and finally neutralization assays. Please read our previous blog post, “Why measurement of viral infectivity matters and how to improve it” to learn more.
Laser Force Cytology (LFC) makes use of a combination of optical force and microfluidics to obtain data on the biophysical and biochemical properties of individual cells, while simultaneously capturing high-resolution images and video of each cell. Using these data enables the rapid and accurate measurement of viral infection in cells. The use of this capability is incredibly valuable for bioprocess optimization and scale-up, where currently the measurement of viral loads can take many days, requires significant skilled labor, and can be difficult to standardize and error prone. LFC is a quantitative method that removes subjectivity, automates analysis, and reduces the time required. For in-process measurement, such as sampling from a bioreactor, LFC can produce accurate infectivity results in 5-10 minutes versus days to weeks using conventional techniques.
To demonstrate the potential of using Laser Force Cytology for cell-based manufacturing, Vero cells were infected with influenza virus and analyzed 1-day post infection at a multiplicity of infection (MOI) of 0.02. Uninfected cells had a higher average velocity than the infected cells, indicating an increase in optical force upon infection. Besides velocity, other biophysical and biochemically relevant parameters were measured for each cell such as: size, eccentricity and deformability. Multiple images were also taken of each cell, thereby providing additional image analysis data for each cell as well as the population as a whole.
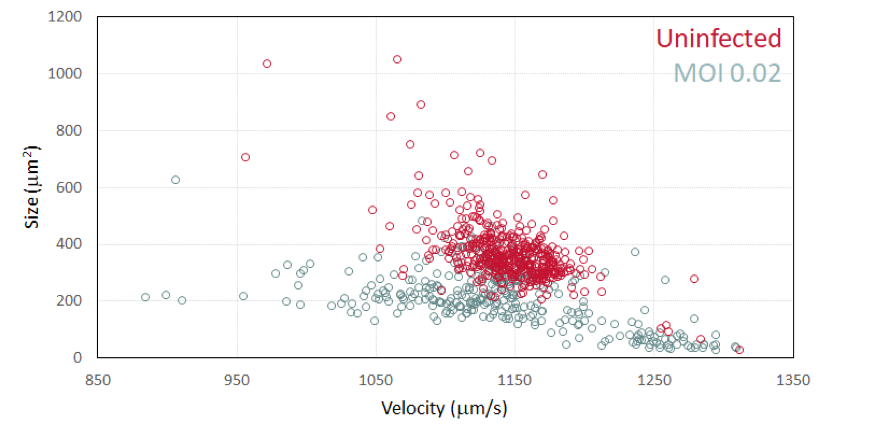
Changes were seen in both the velocity (optical force) and eccentricity (shape) of Vero cells upon influenza infection. A similar trend was previously reported for pseudorabies virus infection in Vero cells (5). For additional data and more detailed explanation of our results, please refer to our tech note on “Laser Force Cytology Monitoring of Influenza Virus Infection”. Radiance could reduce the development time, cost, and complexity of flu vaccine manufacturing, thus making cell culture-based production vs. egg-based production more widespread, potentially improving vaccine effectiveness.
References:
1. Center for Disease Control and Prevention. [Online] February 17, 2018. [Cited: February 26, 2018.]
2. Liu, Angus. FiercePharma. [Online] December 12, 2017. [Cited: February 26, 2018.]
3. Sagonowsky, Eric. FiercePharma. [Online] November 7, 2017. [Cited: February 26, 2018.]
4. H3N2 mutation in last year’s flu vaccine responsible for lowered efficacy. Medical Xpress. [Online] Perelman School of Medicine at the University of Pennsylvania, November 6, 2017. [Cited: February 26, 2018.]
5. Label free detection of pseudorabies virus infection in Vero cells using laser force analysis. Hebert CG, Hart SJ, Terray A. : Analyst, 2014. 139(6):1472-8.


