Posted Keya Rodrigues, MS, Quality Assurance Bioengineer & Margaret McCoy, PhD, JD, Executive Director, Assay Development and Regulatory Affairs
Chinese Hamster Ovary (CHO) cells and other mammalian cells are commonly used as a cell culture line for biomanufacturing proteins of interest. When put under osmotic stress, these cells often display higher specific productivity (1) (2). Interestingly, with greater periods of osmotic stress, there is an increase in specific productivity but also changes in cell morphology, such as variation in cell size (3). A closer examination of cells under osmotic stress may help biomanufacturers understand the parameters for increased specific productivity and the less desirable cellular defense characteristics, leading to the identification of preferred physiological conditions for protein production.
An interesting paper discussing the matter of cell size dynamics is one in which Kiehl et al studied the cell culture populations of two CHO cell lines under osmotic stress with the use of a BD LSRII® flow cytometer, an Innovatis’ Cedex cell analyzer and Beckman Coulter’s Vi-CellTM (3). The results of the flow cytometric and Cedex analysis was the identification of two unique subpopulations with varying cell diameters when placed under different stress levels (3). An expected larger subpopulation comprised of live cells, and a unique smaller and more disperse subpopulation, which was assumed to be composed of dead cells and debris, were observed (3). To further examine this phenomenon, studies were carried out on a CHO A0 cell line culture under hyperosmotic stress, a subsequent reduction in cell viability was observed with the use of two analytical modalities (Vi-CellTM and BD LSRII®). The smaller subpopulation, that could traditionally be mistaken for non-viable cells, were in fact found to be a subpopulation of viable cells (3). Kiehl et al also made the argument that the common practice of using only the mean cell diameter of a population does not fully describe the entire cell culture dynamic nor enable understanding of other subpopulations that might be present (3). Thus, a non-Gaussian distribution for the diameters of cells was utilized in comparison with Gaussian analysis for a more accurate depiction of the subpopulations (3).
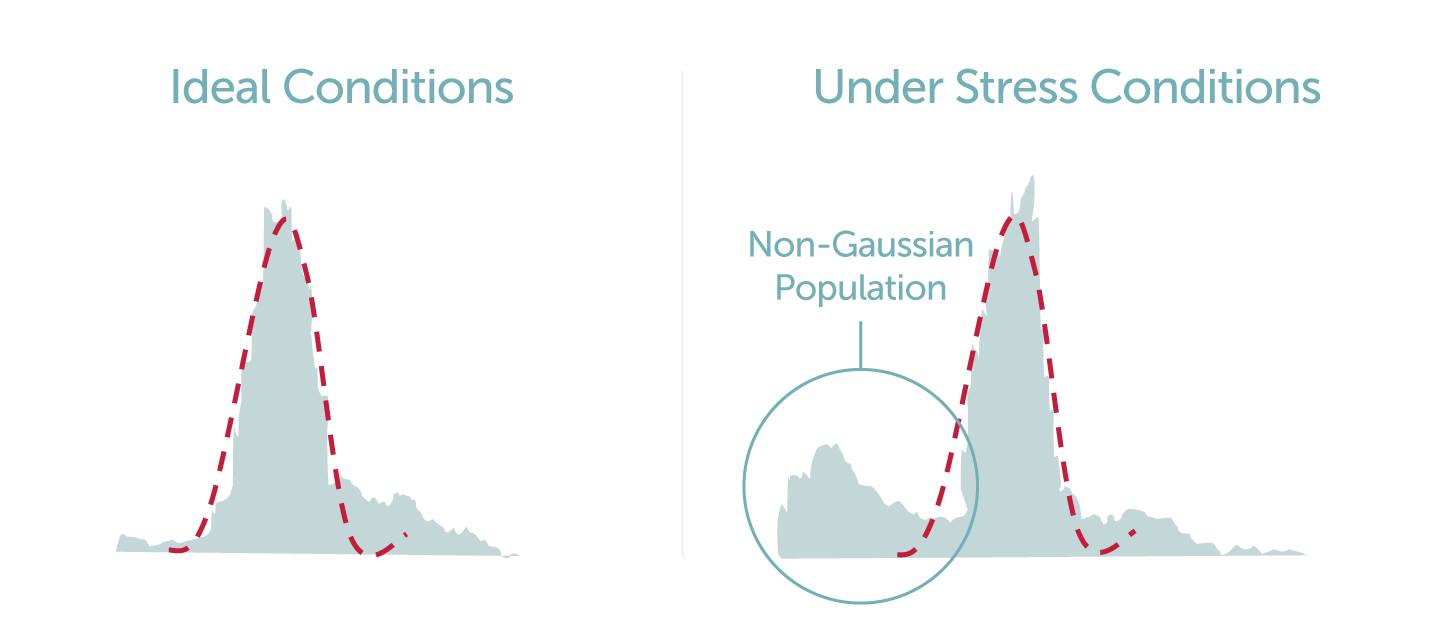
This study has highlighted the need for a detailed and direct method for observing the morphological changes occurring across CHO cell culture populations under varying experimental conditions. LumaCyte’s label-free single cell analysis and sorting instrument, Radiance, is a high-content microfluidic device that enables scientists and researchers to characterize and sort individual cells of interest based upon their intrinsic cellular properties and dynamics using fluidic and optical forces. Radiance can directly quantify samples under varying conditions and measure the biophysical and biochemical cellular properties including cell size, volume, eccentricity, granularity, deformability, effective refractive index, and parameter standard deviations across cell populations. Additional features of Radiance include: cells remain viable for further growth and analysis, the ability to sort cell populations of interest, diverse multivariate data streams to characterize cells, and small sample size requirements as low as 50 microL. The system includes a comprehensive IlluminateTM software suite that includes a robust, intuitive interface to manage the instrument and data analysis.
References
1. Substantial overproduction of antibodies by applying osmotic pressure and sodium butyrate. Oh SKW, Vig P, Chua F, Teo WK, Yap MGS. s.l. : Biotechnol Bioeng, 1993, Vol. 42. 601-610.
2. Effect of medium osmolarity on hybridoma growth, metabolism, and antibody production. Ozturk SS, Palsson BO. s.l. : Biotechnol Bioeng, 1991, Vol. 37. 989-993.
3. Observations of cell size dynamics under osmotic stress. Thomas R. Kiehl, Duan Shen, Sarwat F. Khattak, Zheng Jian Li, Susan T. Sharfstein. 1552-4930, s.l. : Wiley Subscription Services, Inc., A Wiley Company, 2011, Vol. 79A, pp. 560-569.