T-Cell Activation and Cell Killing Analytics using Laser Force Cytology™
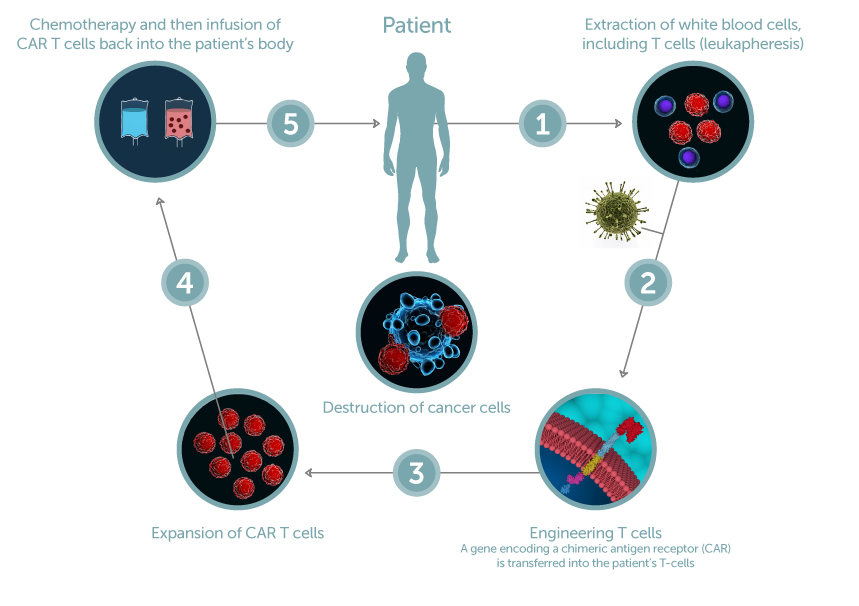
Gene modification cell therapy involves removing cells from a patient and genetically modifying them to introduce a new gene or correct a faulty existing gene. Chimeric antigen receptor (CAR)-T cell therapy is a key example, outlined in the figure on the left. First, T cells are removed from the patient (1) and can undergo qualification/testing which may predict outcome and guide the biomanufacturing process (1). The cells are then genetically modified through delivery of a new gene using a viral vector (2). This gene modification reprograms the T cells to recognize and attack a certain target cancer cell, thereby promoting specific cancer cell destruction and clearance. The next step in the process is to expand (3) or activate and expand/grow the modified T-cells into a large enough quantity, followed by potency testing and formulation (4) prior to infusing the modified cells into the patient (5) to treat their disease, in this case cancer.
Monitoring T-cell activation poses a critical challenge in cell therapies like CAR-T therapy, where precise control is paramount. Achieving the right balance in activation is challenging, as overstimulation may lead to exhaustion or adverse effects, while under-stimulation can compromise therapeutic efficacy. Developing robust and sensitive monitoring techniques is essential to ensuring optimal T-cell activation, addressing a key hurdle in the advancement and success of cell therapies.
Using Laser Force Cytology™, Radiance® is able to effectively monitor and track critical quality attributes (CQA) and critical process parameters (CPP) within the process, quickly measuring the significant biochemical & biophysical changes to the cells during activation. This provides novel and resourceful insights into the condition of the cells and can correlate to the success or failure of the therapy within the patient.
Apheresis
Post apheresis, LFC™ can be used for qualification and testing of initial patient samples to understand donor-to-donor viability.
Cell Expansion
LFC™ enables real-time monitoring of cell growth, viability, and phenotype, crucial for scaling up production.
Quality Control
LFC™ provides a robust tool for quality control, offering detailed insights into the final product’s characteristics, including viability, functionality, and safety.
Rapid Label-Free Monitoring of T-Cell Activation Analytics using Laser Force Cytology™
Here we present LFC™ as a technology that enables a rapid and sensitive method of monitoring T-cell activation using a combination of optical pressure and microfluidics to measure intrinsic biophysical and biochemical changes in cells. The advanced label-free metrics measured by LFC gives it a distinct advantage over other process monitoring technologies that might require fluorescent labeling or other bias-inducing, time-consuming processing steps.


Detection of Mediated CAR-T Cell Killing
Changes in LFC™ metrics across populations can be used to measure cell killing across donors
The results shown demonstrate an LFC™ based in vitro assay to evaluate the efficacy of the CD19 CAR-Ts to kill target cells. The extent of cell killing of the NALM6 cells as measured by LFC™ is an indication of the potency of the CD19 CAR-T cells. Multiple ratios of Effector:Target cells (CAR-T cells:NALM6 cells) were tested to understand robustness of the killing efficiency across the 5 donors tested. As shown in the bar chart, Donors B and E show the highest killing across all 4 ratios, versus Donors C and D that show comparatievly less effective killing, especially when a lower number of effector CAR-T cells were added. The results demonstrate the capability of Radiance® to measure target cell killing in a rapid and label-free manner.



